EMPAIA International e. V. - Pioneer in Digital Pathology
DOI: https://doi.org/10.47184/tp.2024.01.01The recently founded association EMPAIA International e. V. is a pioneer in collaboration and innovation in digital and computational pathology. Continuing the work of the EMPAIA project (2019–2023), which received public funding from the German Federal Ministry for Economic Affairs and Climate Action, the association brings together a broad range of stakeholders to accelerate progress in AI-assisted diagnostics. With its clear focus on standardization, advocacy, and promoting innovation, EMPAIA International plays a leading role in shaping the future of digital pathology.
Keywords: Digital Pathology, AI, Standardization, Interoperability, Eco-System
Novel AI-supported diagnostics enable unprecedented precision and reproducibility in selecting targeted therapies. AI tools are also essential for reducing the workload and addressing the pressing issue of the decline in the number of pathologists.
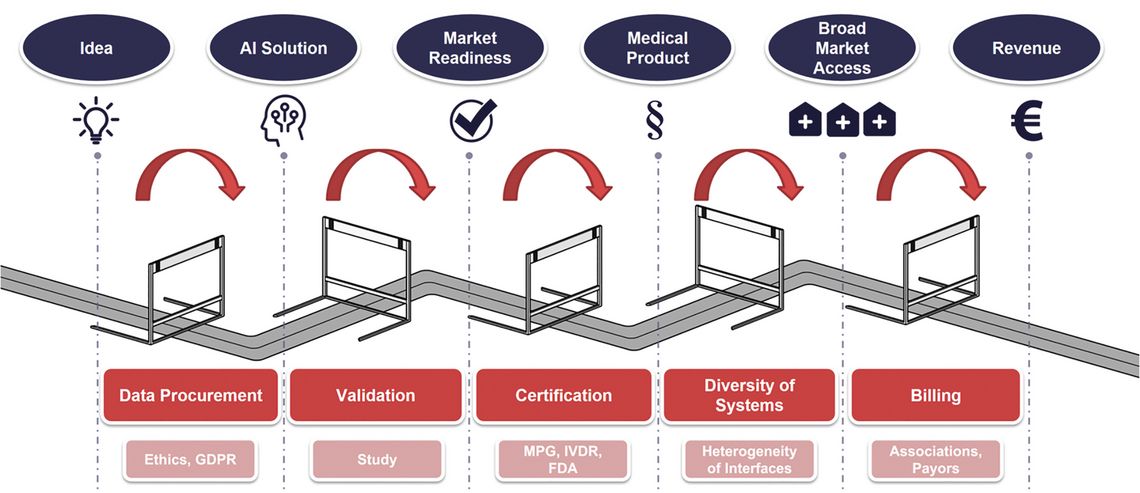
Currently, the use of AI-supported diagnostic procedures is significantly hampered by the lack of standards for integration into the highly heterogeneous IT infrastructure of pathology laboratories. This impedes their evaluation in multi-center studies and subsequent application in diagnostic practice, as well as quality assurance and post-market surveillance.
The integration of the latest scientific findings, fast and reliable diagnostic processes, and the comprehensibility of results for pathologists and patients are crucial for the further advancement of pathology. The complexity of decisions made by pathologists, especially in oncology, has increased enormously. At the same time, increasing digitalization provides them with even more powerful tools to effectively support these decisions, such as AI methods. As the main stakeholders in this field are positioned all over the world, EMPAIA International has developed a comprehensive approach to drive the necessary international developments, standardization, and collaboration.
The EMPAIA approach combines:
- Research, technological innovation, and digitalization
- Standardization
- Validation
- Support of certification
- Post-market surveillance
- Platform-based collaboration
- User-centered approach, including pathologists and patients
In Germany, EMPAIA International is registered as a non-profit organization. In addition to representing the interests of the vaious industry stakeholders, it has also focused on empowering patients and enabling them to exercise their right to their medical data. Through collaboration with patient organizations, we aim to increase patients' awareness of the role of pathology, particularly in oncology, and provide them with access to knowledge and data.
Driving force for cooperation and innovation in pathology
EMPAIA International builds upon the pioneering work of the publicly funded EMPAIA consortium of the German Federal Ministry for Economic Affairs and Climate Action. From the outset, the EMPAIA project has involved a wide range of international stakeholders, including manufacturers of AI solutions/pathology software systems/scanners, diagnosticians from large and small clinical organizations, pharmaceutical companies, and AI researchers. Through collaboration with these stakeholders, EMPAIA has developed guidelines for the commercialization of AI prototypes and validation of AI solutions, establishing an open ecosystem. Another notable project result is the EMPAIA App Interface, an open and vendor-neutral specification that enables the seamless integration of AI solutions into pathology software systems. This standard greatly simplifies the development of interfaces, and EMPAIA International encourages international companies to join the association, adopt EMPAIA interfaces, and continuously improve the standards together. During the project phase, EMPAIA has extensively tested applications using the EMPAIA standard in numerous national and international clinical reference centers to advance the clinical application of AI. This close cooperation with laboratories worldwide will continue within EMPAIA International.
The applied solutions focus on tackling challenges that require the cooperation of two or more stakeholders. The focus is on partnership-based cooperation and mutual exchange. The use of established standards such as DICOM and IHE provides a solid foundation and ensures the seamless integration of new technologies. Neutrality towards all stakeholders is essential to ensure that the different perspectives and needs of all parties are represented.
By adopting this comprehensive approach, the transformation of pathology through digitalization and AI can lead to significant advances in medical science, improved health outcomes and positive contributions to the well-being of pathologists and patients.
Building on its already established network of industry stakeholders, EMPAIA International additionally is also seeking collaborations with patient organizations and policymakers to ensure the development of inclusive and user-centered solutions and the dismantling of regulatory barriers. One of its goals is to facilitate multidisciplinary collaboration to leverage diverse perspectives and expertise. EMPAIA has identified pharmaceutical companies as the main beneficiaries of successful standardization, as they lack real-life data on the effectiveness of their substances used in precision medicine. EMPAIA International aims to gain its support by developing tools for post-market surveillance including the consideration of corresponding indicators in the standardization process. Like all players, membership offers them the opportunity to help shape standards for the integration of AI-supported diagnostic procedures and ensure that their specific requirements are considered.
By bringing together all relevant players from industry, diagnostic practice, and research, the association offers a comprehensive network that contributes to the advancement of digital pathology. Three committees will be established, focusing on legal, validation, and patient empowerment. EMPAIA International is looking for companies interested in joining this network and for individuals who would like to take up an active role in one or more of the committees.
In summary, EMPAIA International e. V. plays a leading role in promoting cooperation and innovation in digital pathology. By working with various interest groups and developing standards, the association makes significant contributions to the further development of this field. Membership allows companies to help shape standards and benefit from a broad network.